TOXICOLOGY
Created to ensure customers can detect new drug-use trends and keep up with a rapidly changing illicit drug market, the testing panel spans approximately 50* drugs and metabolites, including New Psychoactive Substances (NPS).
Verum Gives You
- Provision of previously unavailable data to feed into treatment plans
- Faster treatment decisions by uncovering multi-drug use with one test
- Cost savings from refined treatment plans leading to targeted resource allocation
- Streamlined processes for testing removing the need to screen and confirm
- Ability to identify prescription compliance or misuse
- Unequivocal results from a broad drug panel
Informed Decisions
This wider panel provides more data to make informed decisions to minimise the risk when prescribing/determining treatment in order to improve donor safety. All of this with just one sample, one step and one panel, leading to faster treatment decisions.
Drug and metabolites available for testing with Verum approximately
Increase in drug detections with Verum in comparison to traditional tests
Drugs and/or metabolites detected were previously not available using traditional laboratory tests
Increase in number of positive samples
Of samples were positive for at least one drug or metabolite
Streamlined Process With One Step Testing
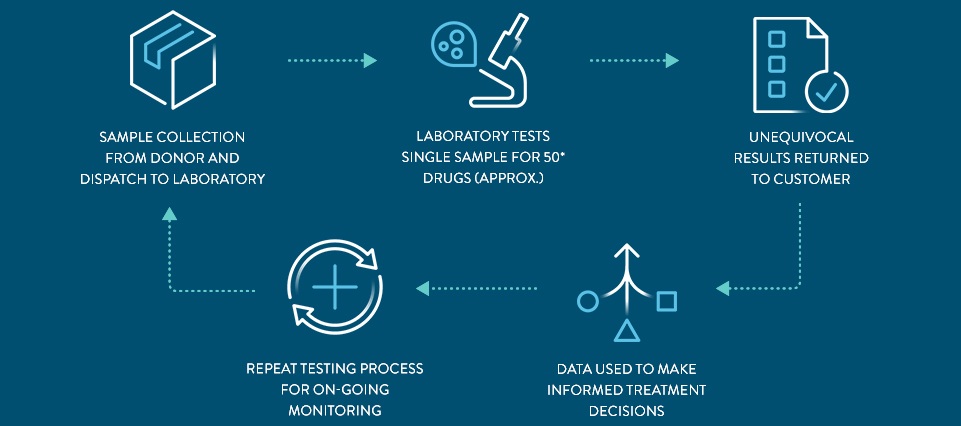
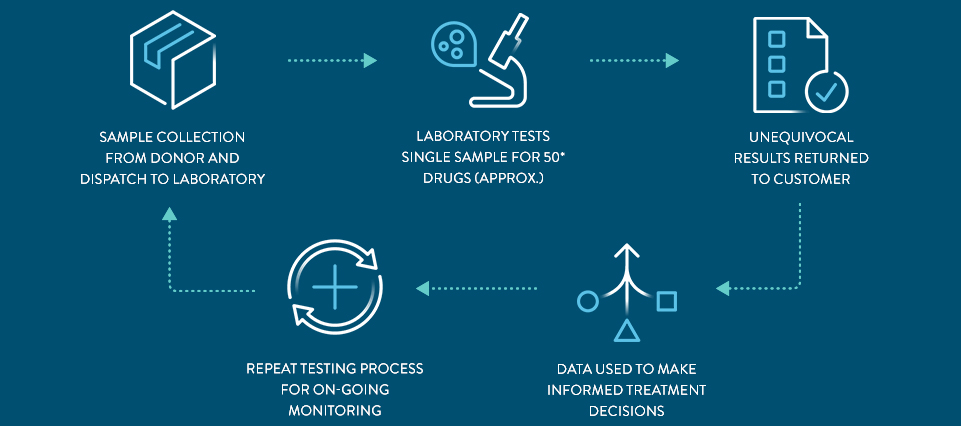

THE PANEL includes approximately 50* drugs within the following drug groups:
Stimulants |
Opiates |
Illicit sedatives and opioids |
Cannabinoids |
Gabapentinoids and prescription only sedatives |
Prescription only sedatives and opioids |
Antidepressants/antipsychotics |
All Data You Need Upfront For Treatment Planning
Get in touch to discuss how Verum can benefit your business.
References
The percentages and numbers displayed in the ‘Informed Decision’ section of this page are generated from data for oral fluid samples received at our laboratory and testing for the Verum Panel between March 2019 and February 2020.
*Refer to Oral Fluid Technical Specifications for most current panel.




