TOXICOLOGY
Testing Explained
Testing can be a confusing business but it doesn't need to be. This section explains key concepts in jargon free, easy to understand English. If you would like more information on a particular topic please contact us.
Drug Abbreviations
Drug testing kits often use an abbreviated drug name rather than listing it in full. This is because space on product packaging and labelling is limited, and listing the full name of ten or more drugs can take up quite a lot of room.
Here is a list of the common drug abbreviations used in the drug testing industry:
Abbreviations
| Abbreviation | Drug Name |
| AMP | Amphetamine |
| BAR | Barbiturates |
| BUP | Buprenorphine |
| BZO | Benzodiazepines |
| COC | Cocaine |
| COT | Cotinine |
| EDDP | EDDP (2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine) |
| FTY | Fentanyl |
| KET | Ketamine |
| MDMA/XTC | MDMA (Methylenedioxymethamphetamine)/Ecstasy |
| MTD | Methadone |
| MET/MAMP | Methamphetamine |
| MOP | Morphine |
| OPI | Opiates |
| OXY | Oxycodone |
| PCP | Phencyclidine |
| PPX | Propoxyphene |
| TCA | Tricyclic Antidepressants |
| THC | Cannabis |
| TRA | Tramadol |
Screening
Modern screening methods are designed to rapidly identify a sample as either negative or presumptive positive if the substance is above a set cut-off concentration. Negative samples are deemed to not require further investigation, whilst samples that are presumed to be positive will require testing by a different analytical technique, known as a confirmation test.
Our screening methods are based on established immunoassay technology and can be conducted in a laboratory or with a rapid diagnostic test at the point of care. Immunoassays use antibodies as reagents to measure the amount of a particular drug or drug group in the sample.
Antibodies bind to the drug by recognising a distinct three dimensional shape. In simplest terms this can be thought of as a key in a lock. The key (drug) has a distinct shape which will only fit into a suitably matched lock (antibody). This concept is shown in figure 1, where each coloured block is a different drug (with a different shape) reacting to a matching antibody.
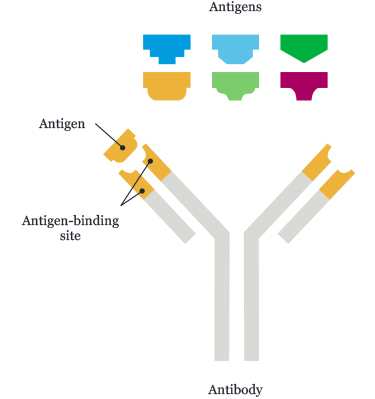
Figure 1: A stylised representation of an antibody recognising one of the shapes. Notice that each shape will only bind to one antibody.
Cut-Off Levels
One of the key concepts within drug testing is the application of a cut-off level. This is the point which segregates a test result as being either positive or negative.
For drug screening tests, a cut-off is chosen that will optimise drug detection but minimise the number of false positive results. It is important to note that a negative sample doesn’t mean that it is drug free; it might contain a drug at an amount that is lower than the defined cut-off.
If a drug test is reported as screen positive or presumptive positive, this merely shows a response, which is usually because a drug is present. It cannot show how much drug was taken or be correlated to any degree of impairment. As this is a screening tool, all presumptive positives require a confirmation test.
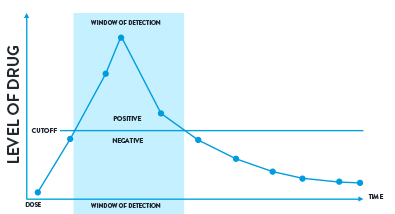

Figure 2: A visual representation of a cut-off level and window of detection.
Windows of Detection
The window of detection is the time that a drug can be detected in a biological sample above a specified cut-off for the test being performed. There is a period of time immediately before and after this detection window when the drug will be present in the sample but at an amount below the cut-off.
Tests are designed to be drug or drug group specific and the cut-off level has been determined to optimise detection without giving false positives.
Drugs are detected in oral fluid either from direct deposition in the mouth or by transfer from the blood stream following ingestion and absorption. Any drugs present in the bloodstream are metabolised in the liver before being excreted in the urine. This process results in drugs appearing in the urine later than oral fluid. The drugs are detectable for a significantly longer time in urine than in oral fluid.
The actual time a drug will remain detectable in a sample will depend on some or all of the following:
- The amount of drug taken
- How frequently the drug is taken
- The nature of the drug itself
- An individual’s metabolism and general health
- The amount of fluids taken since taking the drug
- The amount of exercise taken since taking the drug
- Genetic variations that affect a response to drugs
For example if a person smoked a single spliff the cannabis could remain detectable in urine for no more than 2–3 days and may even be as short as one day depending on the strength of cannabis. However, if their cannabis use is habitual and heavy it is stored in the fatty tissues, resulting in a much wider detection window (sometimes up to 30 days).
The data supplied below acts as a guideline and should be interpreted very carefully due to the large number of factors that can influence the amount of drug over time. The quoted windows of detection indicate how long a drug can be detected. This doesn’t indicate that the drug will be detected for this long in all cases.
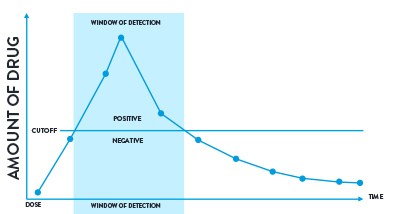
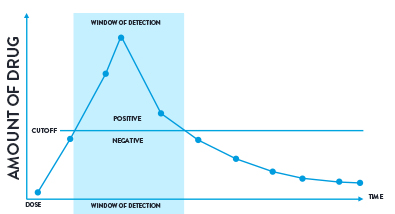

Figure 3: A visual representation of a cut-off level and window of detection.
Oral Fluid
The shortest window of detection is found with oral fluid.
| Drug | Detection Window |
| Cocaine | up to 24 hours |
Benzodiazepines | up to 24 hours |
| Cannabinoids (THC) | up to 24 hours |
| Methamphetamines | up to 24 hours |
| Opiates - Morphine - Codeine | up to 1-2 days - up to 24 hours - up to 1-2 days |
| Amphetamine | up to 1-2 days |
| Buprenorphine | up to 1-2 days |
| Ketamine | up to 1-2 days |
| Methadone | up to 1-2 days |
Urine
Drugs can be detected in urine for longer, and in consequence the window of detection for some drugs can be affected by whether their effects are short or long acting.
| Drug | Detection Window |
| Benzodiazepines - Ultra short acting - Short acting - Intermediate acting - Long acting | - up to 12 hours - up to 1 day - up to 2-4 days - up to 7 days |
Barbiturates | - up to 1-2 days - up to 7 days |
| Methadone | up to 1-2 days |
| Amphetamines | up to 1-3 days |
| Methamphetamines | up to 1-3 days |
| Buprenorphine/Subutex Analgesic - Therapeutic dose - Maintenance dose | - up to 1-3 days - up to 10-12 days |
| Cannabinoids (THC) | up to 1-4 days |
| Cocaine | up to 2-3 days |
| Opiates | up to 2-3 days |
| Ketamine | up to 3-5 days |
| Tramadol | up to 3-5 days |
Hair
Hair provides a historic record of drug use, and detection windows are based entirely on hair length. It takes about 14 days for the drugs to appear in the hair shaft, and moving away from the scalp, every 1cm of hair length approximates to a one month window of detection.
Cross Reactivity
A screening test uses established immunoassay technology to measure the amount of a particular drug or drug group in the sample. Antibodies bind to the drug by recognising a distinct three dimensional shape. In simplest terms this can be thought of as a key in a lock. The key (drug) has a distinct shape which will only fit into a suitably matched lock (antibody). Once the antibody is bound to the drug a colour change occurs, the greater the intensity of this colour change the larger the volume of the drug.
Some drug families share a common shape and so an antibody raised to recognise a drug within that group will detect or cross react with all of the drugs within the family.
There are many hundreds of thousands of drugs available worldwide, many of which are broken down by the body into drug metabolites. With so many over-the-counter (OTC) and prescription only medicines (POM) available, there is a possibility that some of these drugs and their metabolites might have a similar 3-dimensional shape to other drugs and will be detected by the immunoassay. For this reason it is standard practice to perform a confirmation test and request details of a donor’s declared medication after a presumptive positive screening result.



Figure 4: A drug family can share a common shape to which an antibody will bind. Here four different drugs share a triangular section and therefore bind to the top portion of the antibody.
Confirmation
A confirmation test is able to definitively detect individual compounds by matching them to a commercially prepared reference standard using one of the following:
- GC-MS (Gas Chromatography – Mass Spectrometry)
- GC-MS/MS (Gas Chromatography – Tandem Mass Spectrometry)
- LC-MS/MS (Liquid Chromatography – Tandem Mass Spectrometry)
Confirmation tests provide qualitative results of drugs and/or drug metabolites present in the sample.
Drugs are rapidly metabolised in the body to aid excretion and detection of the resulting metabolites indicates drug use by the donor. Sometimes it is only the metabolites that are positive in a confirmation test. For example, heroin is rapidly broken down to 6-monoacetylmorphine (6-MAM) and cocaine is rapidly broken down to benzoylecgonine. Detection of these metabolites demonstrates use of heroin and cocaine respectively. Only a confirmation test would allow the laboratory to make the distinction between illicit, prescription medicines or over the counter medication.
Confirmation results are expressed as positive or negative and are legally defensible.
CHAIN OF CUSTODY
The results of a drug or alcohol test can have life-changing ramifications for a donor. For this reason, we handle every single sample with painstaking attention to detail as we understand there is a life behind each result. Following a strict chain of custody procedure gives peace of mind to both our customers and donors.
The chain of custody is the chronological documentation or paper trail, showing the collection, transfer, receipt, analysis, storage, and disposal of the sample. This ensures that any results we report relate beyond all reasonable doubt to a particular individual.
If the results should ever need to be used in court as evidence, our chain of custody records are fully defensible and can disprove any allegations of tampering or misconduct.
Our Process
SAMPLE COLLECTION
We firstly check the donor’s photographic ID so that we can be sure who is providing the sample. We then obtain their consent to test and during the collection we protect against sample adulteration or substitution. Unique barcodes are placed on the sample vials and on the laboratory paperwork so that the sample is tied with the information on the paperwork. Tamper evident seals are also placed on the specimen containers to show if any attempt has been made to remove them.
Both the Collecting Officer and the donor sign the laboratory paperwork before it and the sample are placed into secure packaging for transit to the laboratory. By signing the laboratory paperwork, the donor is confirming that the sealed sample is the sample that was collected from them.
ANALYSIS
Once the sample arrives at the laboratory, we check for any evidence of tampering and visible signs of contamination. The barcodes of the paperwork and samples are scanned into our LIMS (Laboratory Information Management System). The LIMS then tracks the sample during analysis, using the original sample barcode as a unique identifier at all stages of the process. All associated records for the analysis are retained and available to demonstrate traceability at audit.
REPORTING
Prior to reporting of analytical results a senior member of analytical staff reviews the analytical results and sample information before authorising the result for reporting. If a medical review is required a Medical Review Officer will conduct the review using the original sample barcode to identify the sample.
These processes ensure that a sample is clearly identified from sample collection, receipt at the laboratory, sample analysis, through to result reporting and medical review, ensuring that the final result has a traceable link to the individual from whom the sample was collected.
Medical Review Officer
A Medical Review Officer (MRO) provides independent and expert scrutiny of positive results under medical confidentiality. In the case of a positive laboratory result, the MRO must determine whether this indicates the use of drugs in contravention of a company’s rules, or whether there may be a legitimate explanation.
The medical review process protects individuals from wrongful accusations when legitimate medications cause a positive result, for example, because they contain over-the-counter opiates. Not only are wrongful accusations distressing for the individual but can also lead to legal action against the employer along with adverse publicity. Therefore we strongly advise all clients testing their workforce to use a medical review process.
Expert Witness Report
Using an experienced provider who supplies thousands of reports each year will give you the peace of mind of a reliable interpretation of the results. An Expert Witness Report will provide you with professional, objective evidence suitable for use in court.
An Expert Witness Report provides an interpretation of the results of drug and/or alcohol tests, and explains the testing procedure from sample collection through to test results.
It contains information regarding the chain of custody in place to maintain sample integrity, a detailed description of the testing procedure and whether or not the substances tested for were detected. Test results need professional interpretation to enable a full understanding of the results, to put them into context and ensure they are used correctly. Advice from our experienced Toxicologists ensures that you are fully informed.
REPORTS WRITTEN TO YOUR REQUIREMENTS
Our reports are tailored to the relevant court as directed by you. By indicating on our request form whether you require a report for family or civil court, it will be written to meet either:
Ministry of Justice Civil Procedure Rules Part 35 Practice Direction 35 Experts and Assessors
Or
Ministry of Justice Family Procedure Rules Part 25 Expert and Assessors and Practice Directions 25A Experts and Assessors
COURT ATTENDANCE
Our Expert Witnesses are available to attend court to explain the testing procedure, their interpretation of results and the chain of custody as required.
Programme Management
When implementing drug and alcohol testing in the workplace it is vital to maintain objectivity. Using a third party to do this allows for unannounced / random testing to be implemented effectively and without employees feeling they are being singled out. In addition to this the administration and logistics of implementing a testing programme can be difficult, especially when trying to work within both international regulations and local legislation, particularly when your organisation is distributed internationally.
An expertly managed programme can guide you through regulations to facilitate a testing programme that is both suitable and legal. Our dedicated Programme Management Team will work with you to organise the collection of samples both as part of your managed programme or in response to an incident. Their guidance can be customised to suit your situation, whether simply responding to your requests or assuming responsibility for management of the whole programme.
Some clients need to evidence their adherence to industry regulations regarding substance misuse during inspections and audits. We issue you with a certificate of registration to evidence your participation in a drug and alcohol testing programme. We also issue certificates of completion to you at the end of the year, once all testing has been completed.

