TOXICOLOGY
Quantisal™ Oral Fluid Collection Devices
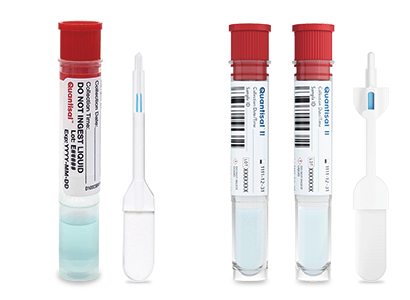


Quantisal™ collection devices are widely used for oral fluid drug testing, combining unique scientific advantages with the efficiency of a simple, convenient specimen collection. Quantisal offers an innovative testing format that allows samples to be collected anytime, anywhere.
Benefits
Collect samples anywhere, protecting against specimen tampering or adulteration
Excellent indication of recent drug use with parent drugs present in higher concentration than metabolites
Non-invasive, gender-neutral specimen collections
Non-regulated and Regulated testing programs
When choosing the test sample, it is important to establish the purpose and intent of the testing program.
NON-REGULATED
A drug testing program that does not include Department of Transportation (DOT) or other federally regulated employees. The program may mirror DOT panels and cutoffs.
Examples include:
- Pre-employment screening, post-accident screening
- Drug treatment, pain management
REGULATED
Regulated drug testing programs follow strict guidelines and are required to meet federal standards for employment testing in federally approved laboratories ensuring consistency and reliability in results.
Examples include:
- Department of Transportation (DOT)
- Substance Abuse and Mental Health Services (SAMHSA)
- Other federal agencies
What does an oral fluid drug test detect?
The available testing menus differ between non-regulated and regulated testing programs.
Non-Regulated
- 6-Acetylmorphine
- (+)-Amphetamines
- Benzodiazepines
- Buprenorphine
- Cannabinoids
- Cocaine/Benzoylecgonine
- Ethyl Alcohol
- Hydrocodone
- Marijuana (THC)
- MDMA
- Methadone
- (+)-Methamphetamine
- Opiates
- Oxycodone
- Phencyclidine (PCP)
- Tramadol
Regulated
- 6-Acetylmorphine
- Amphetamines
- Benzoylecgonine
- Cannabinoids
- Hydrocodone
- MDMA
- Methamphetamine
- Opiates
- Oxycodone
- PCP

In today’s fast-paced, safety-sensitive industries, the need for accurate, efficient, and practical drug screening solutions is more critical than ever. That’s why oral fluid drug testing is emerging as a game-changer. Watch our recent webinar for real-world, practical applications of oral fluid screening.
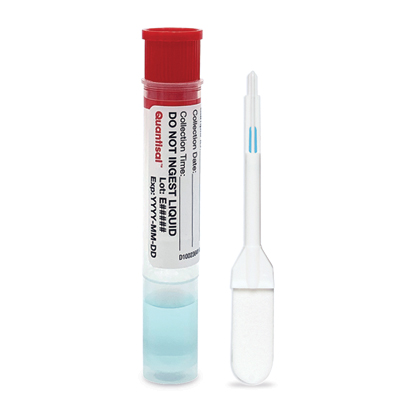
Quantisal™ Oral Fluid Collection Device
The Quantisal™ Oral Fluid Collection Device is widely used for oral fluid drug testing in workplace or pain and addiction practice settings. It allows for a simple noninvasive observed collection to avoid issues with sample adulteration or donor privacy.
Features:
- Collects 1 mL +10% oral fluid
- Excellent indication of recent drug use1
- Excellent extraction efficiencies
- No artificial stimulants
- Volume adequacy indicator
- Reduces specimen adulteration
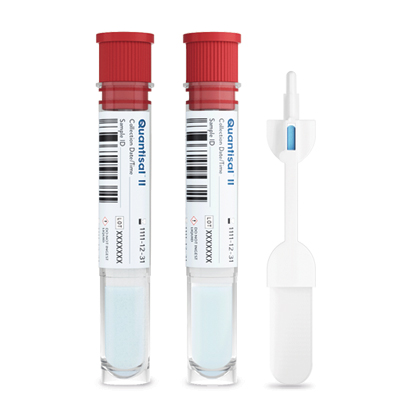
Quantisal™ II Oral Fluid Split Specimen
Collection Device
Quantisal™ II is the first commercially available oral fluid device with split specimen capabilities, offering you the simplicity of collecting two samples with a single device that meets SAMHSA2 and Department of Transport (DOT)3 guidelines.
All the Features of Quantisal plus:
- Simultaneous collection of A and B specimens
- Proprietary FDA 510(k) cleared device
Helpful Documents
Product Documents
References
- White, R, Moore, C. Detection of Drugs and Their Metabolites in Oral Fluid. Elsevier and RTI International, 2018
- Substance Abuse and Mental Health Services Administration (SAMHSA)
- Department of Transportation (DOT), Federal Aviation Administration, 14 CFR Part 120, Procedures for Transportation Workplace Drug and Alcohol Testing Programs: Addition of Oral Fluid Specimen Testing for Drugs, Federal Register Vol. 88 No. 84, May 02, 2023

